Menu
≡
╳
- Home
- About Us
-
Group Companies
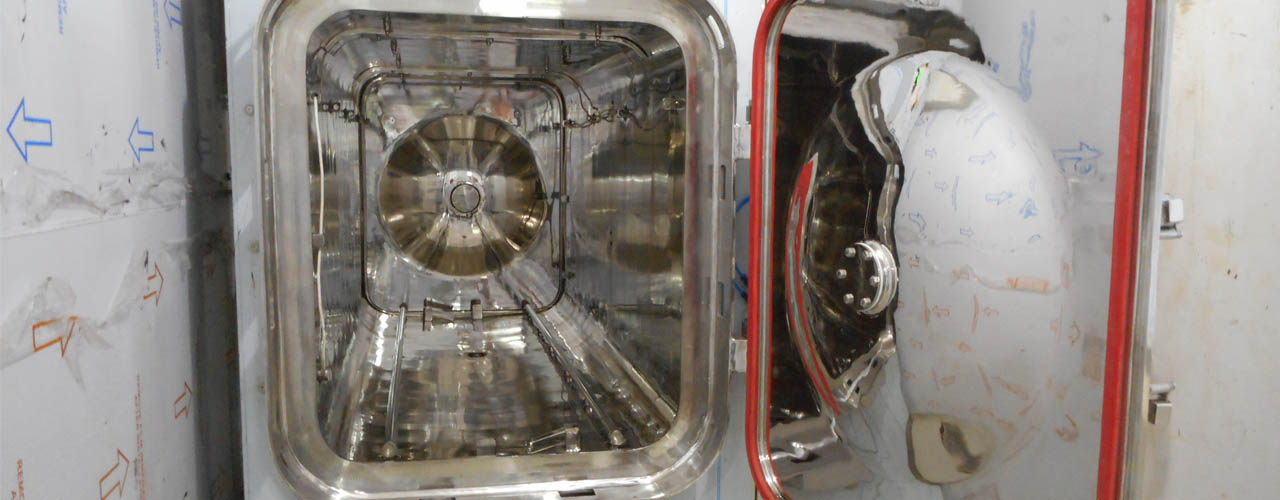
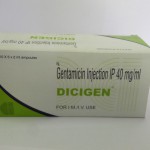
- Agrawal Medicaments Pvt. Ltd. (AMPL)
- Agrawal Minerals (Goa) Pvt. Ltd.
- Agrawal Renewable Energy Pvt. Ltd.
- Agrawal Solar Power Ventures (Rajasthan) Pvt. Ltd.
- Medizest Pharmaceutical Pvt. Ltd.
- AryaVaan Pharmaceuticals
- Bloomz International School
- Ferromar Shipping Pvt. Ltd.
- DCI Pharmaceuticals Pvt. Ltd.
- Gangadhar Narsingdas Agrawal (HUF)
- Kayji Real Estate Pvt. Ltd.
- Our business
- Ongoing projects
- Our Clients
- Media Forum
- Contact Us
- Login